
Devenez membre d'Incathlab et bénéficiez d'un accès complet !
Inscription Connexion
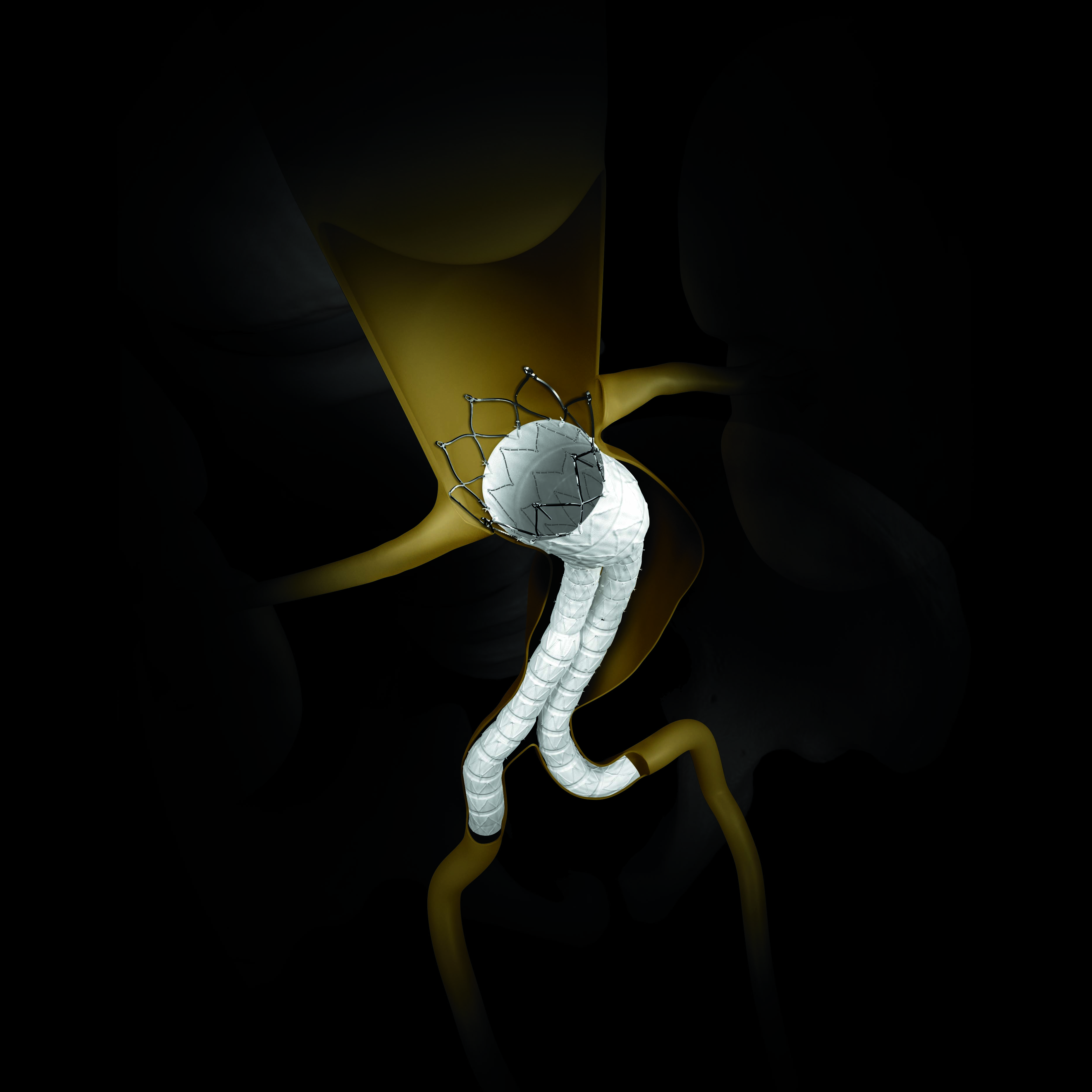
In the last twenty years, endovascular technology has revolutionized the management of patients with abdominal aortic aneurysm (AAA) because of its less invasiveness.
Endovascular stent grafting is a newer form of treatment for Abdominal Aortic Aneurysm that is less invasive than open surgery. The technology has evolved from large profile endoprothesis but requires open-surgery to low profile endoprothesis implantable percutaneously (PEVAR).
Furthermore, the development of different type of endoprothesis and extension has allowed the treatment of more complex abdominal aorta aneurysm.
This second websymposium describes the latest low profile endovascular prothesis and its use by our Italian colleagues.
Program (GMT +1)
| 16:00 | Introduction – Prof. Torsello |
| 16:10 | 1st case in the box – "52 mm asymptomatic AAA treated with incraft Endograft" - Dr. Schwindt |
| 16:30 | The Durability and long term data for low profile AAA stent grafts - Dr. Schwindt |
| 16:40 | 2nd case in the box – "Complex EVAR in tortuous anatomy and exclusion of hypogastric artery aneurysm with the low profile Incraft prothesis" - Dr. Schwindt |
| 17:05 | The use of low profile AAA stent graft in our daily practice - Prof. Torsello |
| 17:15 | 3rd case in the box – Prof. Torsello |
| 17:35 | Q&A final discussion and debate - Prof. Torsello, Dr Schwindt |
| 18:00 | Take home messages - end of the webinar |
Educational objectives
- Discuss current use of low profile AAA stent grafts
- Provide platform for discussion of the patients’ outcomes and population treated with low profile AAA stent grafts
- Demonstrate advanced techniques in the treatment of aortic disease
- Demonstrate new tools available
- Provide platform for discussion and mutual learning
Audience
- Endovascular specialists (Vascular Surgeons, Interventional Radiologists, Interventional Angiologists and Interventional Cardiologists)
- Referring physicians of patients with vascular disease
IMPORTANT NOTICE
This webinar is an educational event supported by Cordis Corporation, and is intended for Healthcare Professionals in Europe Middle East and Africa.
The use of the INCRAFT® AAA Stent-Graft System requires that physicians be specially trained in endovascular abdominal aortic aneurysm repair techniques, including experience with high resolution fluoroscopy and radiation safety. Cordis Corporation will provide training specific to the INCRAFT® AAA Stent-Graft System.
The INCRAFT® System is currently approved for investigational device use only in the U.S. and Japan and is being studied in a global pivotal clinical study in the U.S. and Japan called the INSPIRATION Trial, which completed enrollment in 2013.
While every effort is made to see that no inaccurate or misleading data, opinions, or statements appear in this webinar, Cordis Corporation wish to make it clear that material contained in the webinar represents independent evaluations and opinions of the authors and contributors. As a consequence, Cordis Corporation accepts no responsibility for the consequences of any such inaccurate or misleading data or statements. Neither do they endorse the content or the use of any drug or medical device in a way that lies outside its current licensed application in any territory. This presentation may include the demonstration of the use of medical devices; it is not intended to be used as a training guide. The steps demonstrated may not be the complete steps of the procedure. Individual preference and experience, as well as patient needs, may dictate variation in procedure steps. Before using any medical device, including those demonstrated or referenced in this webinar, review all relevant package inserts, with particular attention to the indications, contraindications, warnings and precautions, and steps for use of the device.
All content in these presentations is owned by their authors and is protected by worldwide copyright laws. No modification or further reproduction of the content is permitted.
Prof. Torsello, Prof. Kellersmann and Dr. Schwindt are compensated by and presenting on behalf of Cordis.
©2016 Cardinal Health. All Rights Reserved.
CORDIS and the Cordis LOGO is a trademark of Cordis Corporation.
EU1053 10/16
Dernière mise à jour : 13/11/2017




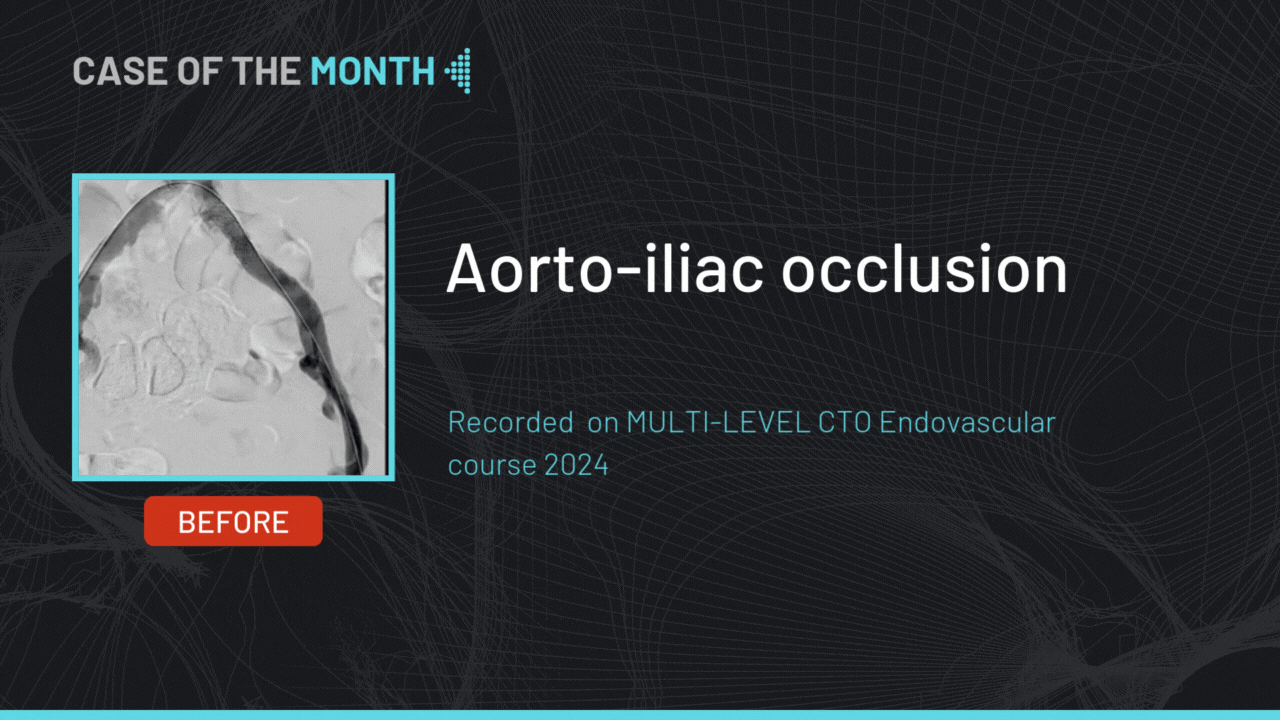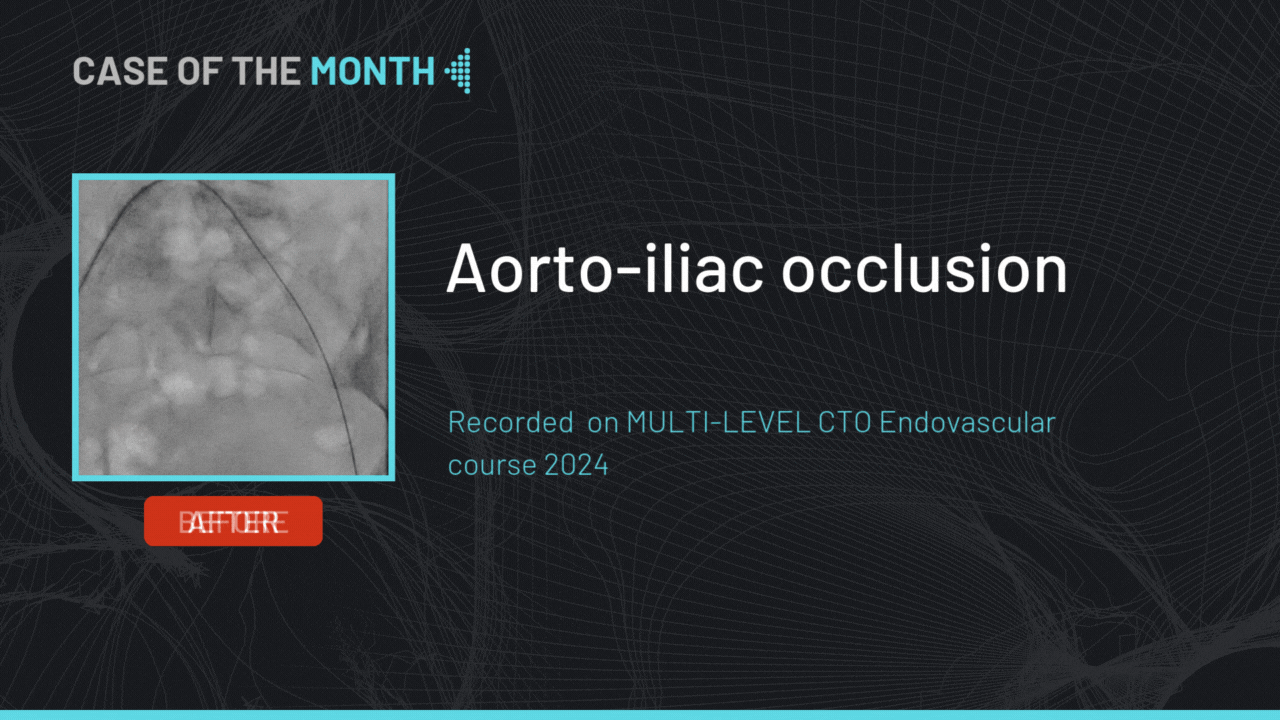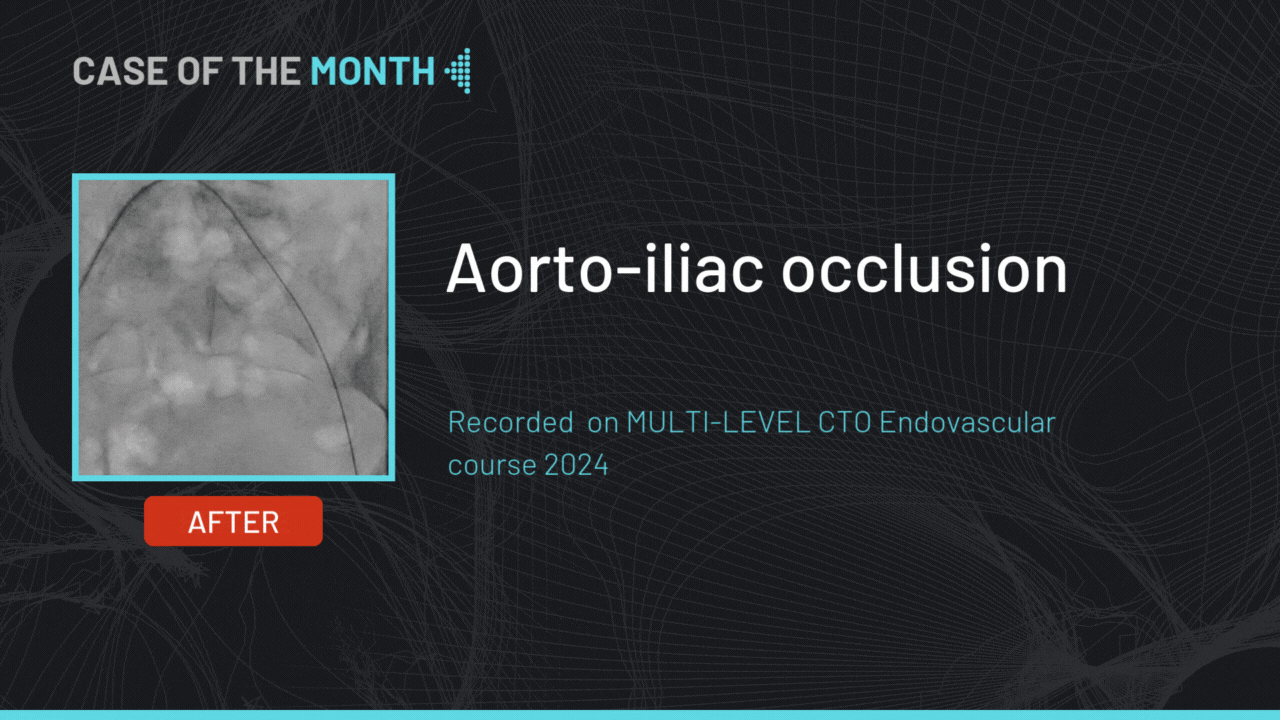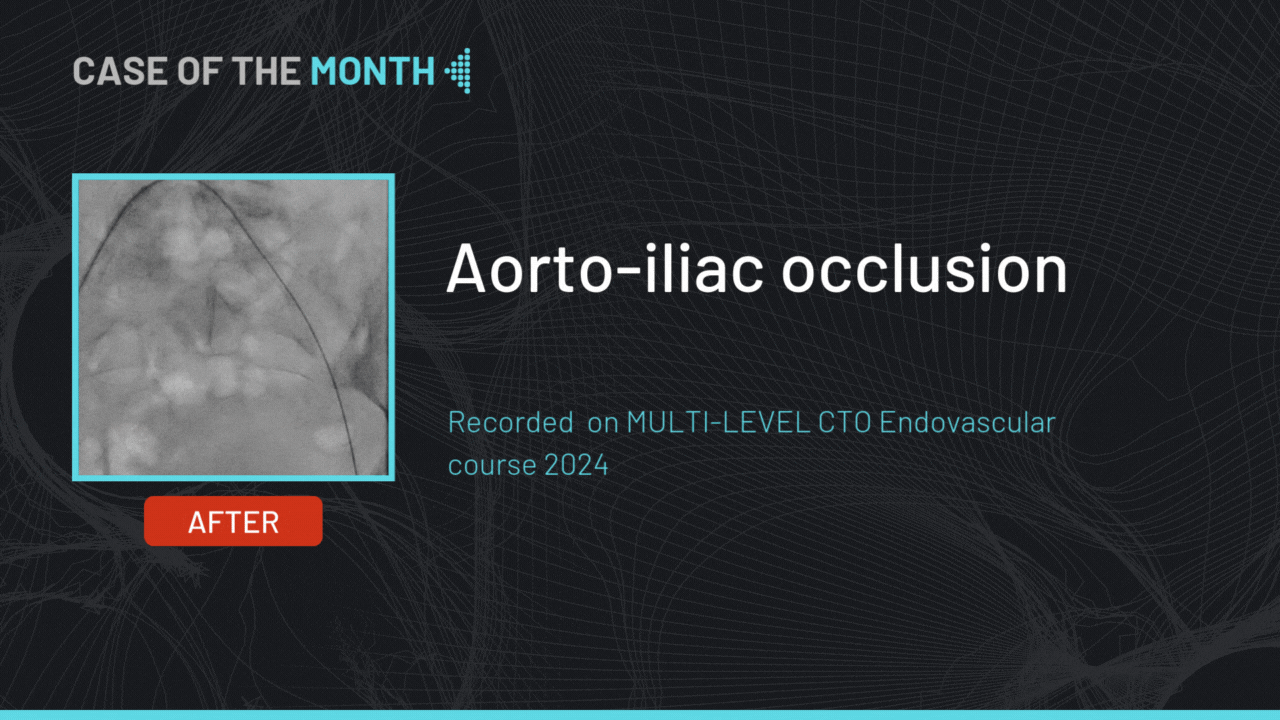


gerson U. Great work! What make is your angio machine and table? What is the failure rate of the pre-closure device?
Paulo P. Dears Drs. Giovanni Torsello and Arne Schwindt, Congratulations for the excellent presentiation ! Regards, Paulo Ocke Reis (Brazil,RJ)
Patricio N. Can you expect a tipe 1 endoleak in this case?
Arne S. Dear Gerson Uanivi, we worked in a Siemens Hybrid OR with a AXIUM ARTIS Machine, The Preclose thechnique has a learning curve, in our experience the success rate of sugeons with 20+ cases is 95%. All the best Arne Schwindt
Arne S. Dear Patricio Navarro, both patients received a post OR CT, Patient one has a type II EL via the IMA, Patient two has a verry small type II EL via lumbar L5, no type IA or B EL were detected. I wish you a happy 2017 Arne Schwindt
Elias K. good evening, i was a few days late watching the video.
For up to 18F puncture sites, would you use one prostar or two perclose devices and why?
Arne S. Dear Dr. K.,
in our setting we use one Prostar device for all large access procedures (EVAR, TEVAR, FEVAR...). The reason is that using one device instead of two is a bit less time consuming. However using two Proglides instead seems to be an alternative. There is one publication showing superiority of Proglide versus Prostar ( if I recall right recently in Eurointervention) for TAVI but you could argue that for cardiologists Priglide is more comfortable because the slipknot is inherited whilst for Prostar it has to be fascilitated by the user. For my taste both strategies are good as long as you got a good training on the device.
All the best
Arne Schwindt