×
Il semble que vous utilisiez une version obsolète de internet explorer. Internet explorer n'est plus supporté par Microsoft depuis fin 2015. Nous vous invitons à utiliser un navigateur plus récent tel que Firefox, Google Chrome ou Microsoft Edge.
My Player placeholder

Devenez membre d'Incathlab et bénéficiez d'un accès complet !
Vous devez être membre pour accéder aux vidéos Incathlab sans limitation. Inscrivez vous gratuitement en moins d'une minute et accédez à tous les services Incathlab ! Vous avez aussi la possibilité de vous connecter directement avec votre compte facebook ou twitter en cliquant sur login en haut à droite du site.
Inscription Connexion
Inscription Connexion
35880 vues
The benefits of lower-profile devices are obvious. Not only can more patients with smaller access vessels be treated without the need for conduit access or bypass graft surgery, but access site complications are lower the smaller the device, and more can be done percutaneously without the need for surgical arteriotomy for access.
Discover in this webinar the ultra-low profile abdominal aortic aneurysm stent graft system, which demonstrated to be easy to use, accurate in placement and effective in navigational challenges.
Program
| 12:30 | Product Review & 5 years Innovation study results - Dr Llaneza |
| 12:45 |
1st Case "Narrow access case" - Panel Discussion Dr Llaneza, Dr del Castro and Dr Alonso |
| 13:15 |
Incraft® Concept: an advantage ONLY for complex access? - Dr del Castro Preserve Hypogastric - IN Situ Sizing Short Necks - Deployment accuracy |
| 13:25 |
2nd Case"Hypogastric preservation case" - Panel Discussion Dr Llaneza, Dr del Castro and Dr Alonso |
| 13:55 | Closing & Remarks |
Educational objectives
- Discuss current use of ultra-low profile Incraft® AAA stent graft systems
- Learn from expert discussion of the patients’ outcomes with low profile Incraft® AAA stent graft system treatment
- Demonstrate advanced techniques in the treatment of aortic disease
- Demonstrate new tools available
- Provide platform for discussion and mutual learning
Bibliography
- INNOVATION: Four-year Safety and Effectiveness of the INCRAFT AAA Stent Graft for Endovascular Repair of Abdominal Aortic Aneurysms. Pratesi G., Pratesi C., Chiesa R., Coppi G., Scheinert D., Brunkwall JS., Van der Meulen S., Torsello G.
- Fast-track endovascular aneurysm repair: rationale and design of the multicenter Least Invasive Fast-Track EVAR (LIFE) registry. Krajcer Z., Ramaiah V., Huetter M.
- The Preclose Technique in Percutaneous Endovascular Aortic Repair: A Systematic Literature Review and Meta-Analysis. Jaffan AA., Prince EA., Hampson CO., Murphy TP.
- A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). Nelson PR., Kracjer Z., Kansal N., Rao V., Bianchi C., Hashemi H., Jones P., Bacharach JM.
Audience
- Endovascular specialists (Vascular Surgeons, Interventional Radiologists, Interventional Angiologists and Interventional Cardiologists)
-
Referring physicians of patients with vascular disease
IMPORTANT NOTICE
This webinar is an educational event supported by Cordis Corporation, and is intended for Healthcare Professionals in Europe Middle East and Africa.
The use of the INCRAFT® AAA Stent-Graft System requires that physicians be specially trained in endovascular abdominal aortic aneurysm repair techniques, including experience with high resolution fluoroscopy and radiation safety. Cordis Corporation will provide training specific to the INCRAFT® AAA Stent-Graft System.
The INCRAFT® System is currently approved for investigational device use only in the U.S. and Japan and is being studied in a global pivotal clinical study in the U.S. and Japan called the INSPIRATION Trial, which completed enrollment in 2013.
While every effort is made to see that no inaccurate or misleading data, opinions, or statements appear in this webinar, Cordis Corporation wish to make it clear that material contained in the webinar represents independent evaluations and opinions of the authors and contributors. As a consequence, Cordis Corporation accepts no responsibility for the consequences of any such inaccurate or misleading data or statements. Neither do they endorse the content or the use of any drug or medical device in a way that lies outside its current licensed application in any territory. This presentation may include the demonstration of the use of medical devices; it is not intended to be used as a training guide. The steps demonstrated may not be the complete steps of the procedure. Individual preference and experience, as well as patient needs, may dictate variation in procedure steps. Before using any medical device, including those demonstrated or referenced in this webinar, review all relevant package inserts, with particular attention to the indications, contraindications, warnings and precautions, and steps for use of the device.
All content in these presentations is owned by their authors and is protected by worldwide copyright laws. No modification or further reproduction of the content is permitted.
Dr Manuel Alonso, Dr Llaneza and Dr Del Castro are compensated by and presenting on behalf of Cordis.
For Healthcare Professionals Only.
©2017 Cardinal Health.
Cordis, Cordis LOGO and INCRAFT are trademarks or registered trademarks of Cardinal Health.
EU2321 05/17
Date du tournage : 24/05/2017
Dernière mise à jour : 29/08/2017
Dernière mise à jour : 29/08/2017
Participer à la discussion
Suggestions
Mercredi 23 octobre 2024 de 12h à 12h30 (GMT+2)
Honolulu : Mercredi 23 octobre 2024 de 00h à 00h30 (GMT+2)
San Francisco : Mercredi 23 octobre 2024 de 03h à 03h30 (GMT+2)
New York : Mercredi 23 octobre 2024 de 06h à 06h30 (GMT+2)
Buenos Aires : Mercredi 23 octobre 2024 de 07h à 07h30 (GMT+2)
Reykjavik : Mercredi 23 octobre 2024 de 10h à 10h30 (GMT+2)
London / Dublin : Mercredi 23 octobre 2024 de 11h à 11h30 (GMT+2)
Paris / Berlin : Mercredi 23 octobre 2024 de 12h à 12h30 (GMT+2)
Istanbul : Mercredi 23 octobre 2024 de 13h à 13h30 (GMT+2)
Moscou / Dubaï : Mercredi 23 octobre 2024 de 14h à 14h30 (GMT+2)
Bangkok : Mercredi 23 octobre 2024 de 17h à 17h30 (GMT+2)
Shanghai : Mercredi 23 octobre 2024 de 18h à 18h30 (GMT+2)
Tokyo : Mercredi 23 octobre 2024 de 19h à 19h30 (GMT+2)
Sydney : Mercredi 23 octobre 2024 de 21h à 21h30 (GMT+2)
Wellington : Mercredi 23 octobre 2024 de 23h à 23h30 (GMT+2)
San Francisco : Mercredi 23 octobre 2024 de 03h à 03h30 (GMT+2)
New York : Mercredi 23 octobre 2024 de 06h à 06h30 (GMT+2)
Buenos Aires : Mercredi 23 octobre 2024 de 07h à 07h30 (GMT+2)
Reykjavik : Mercredi 23 octobre 2024 de 10h à 10h30 (GMT+2)
London / Dublin : Mercredi 23 octobre 2024 de 11h à 11h30 (GMT+2)
Paris / Berlin : Mercredi 23 octobre 2024 de 12h à 12h30 (GMT+2)
Istanbul : Mercredi 23 octobre 2024 de 13h à 13h30 (GMT+2)
Moscou / Dubaï : Mercredi 23 octobre 2024 de 14h à 14h30 (GMT+2)
Bangkok : Mercredi 23 octobre 2024 de 17h à 17h30 (GMT+2)
Shanghai : Mercredi 23 octobre 2024 de 18h à 18h30 (GMT+2)
Tokyo : Mercredi 23 octobre 2024 de 19h à 19h30 (GMT+2)
Sydney : Mercredi 23 octobre 2024 de 21h à 21h30 (GMT+2)
Wellington : Mercredi 23 octobre 2024 de 23h à 23h30 (GMT+2)
The Complex Made Simple: A Novel All-in-One Solution for Infrainguinal CTO
Partager
0
Days
0
Hours
0
Minutes
0
Seconds
Vendredi 4 avril 2025 de 13h à 15h (GMT+2)
Honolulu : Vendredi 4 avril 2025 de 01h à 03h (GMT+2)
San Francisco : Vendredi 4 avril 2025 de 04h à 06h (GMT+2)
New York : Vendredi 4 avril 2025 de 07h à 09h (GMT+2)
Buenos Aires : Vendredi 4 avril 2025 de 08h à 10h (GMT+2)
Reykjavik : Vendredi 4 avril 2025 de 11h à 13h (GMT+2)
London / Dublin : Vendredi 4 avril 2025 de 12h à 14h (GMT+2)
Paris / Berlin : Vendredi 4 avril 2025 de 13h à 15h (GMT+2)
Istanbul : Vendredi 4 avril 2025 de 14h à 16h (GMT+2)
Moscou / Dubaï : Vendredi 4 avril 2025 de 15h à 17h (GMT+2)
Bangkok : Vendredi 4 avril 2025 de 18h à 20h (GMT+2)
Shanghai : Vendredi 4 avril 2025 de 19h à 21h (GMT+2)
Tokyo : Vendredi 4 avril 2025 de 20h à 22h (GMT+2)
Sydney : Vendredi 4 avril 2025 de 22h à 00h (GMT+2)
Wellington : Samedi 5 avril 2025 de 00h à 02h (GMT+2)
San Francisco : Vendredi 4 avril 2025 de 04h à 06h (GMT+2)
New York : Vendredi 4 avril 2025 de 07h à 09h (GMT+2)
Buenos Aires : Vendredi 4 avril 2025 de 08h à 10h (GMT+2)
Reykjavik : Vendredi 4 avril 2025 de 11h à 13h (GMT+2)
London / Dublin : Vendredi 4 avril 2025 de 12h à 14h (GMT+2)
Paris / Berlin : Vendredi 4 avril 2025 de 13h à 15h (GMT+2)
Istanbul : Vendredi 4 avril 2025 de 14h à 16h (GMT+2)
Moscou / Dubaï : Vendredi 4 avril 2025 de 15h à 17h (GMT+2)
Bangkok : Vendredi 4 avril 2025 de 18h à 20h (GMT+2)
Shanghai : Vendredi 4 avril 2025 de 19h à 21h (GMT+2)
Tokyo : Vendredi 4 avril 2025 de 20h à 22h (GMT+2)
Sydney : Vendredi 4 avril 2025 de 22h à 00h (GMT+2)
Wellington : Samedi 5 avril 2025 de 00h à 02h (GMT+2)
The Drug Eluting Technology Show
Partager
Vendredi 23 février 2024 de 11h à 12h30 (GMT+1)
Honolulu : Jeudi 22 février 2024 de 23h à 00h30 (GMT+1)
San Francisco : Vendredi 23 février 2024 de 01h à 02h30 (GMT+1)
New York : Vendredi 23 février 2024 de 04h à 05h30 (GMT+1)
Buenos Aires : Vendredi 23 février 2024 de 06h à 07h30 (GMT+1)
London / Dublin : Vendredi 23 février 2024 de 09h à 10h30 (GMT+1)
Paris / Berlin : Vendredi 23 février 2024 de 10h à 11h30 (GMT+1)
Istanbul : Vendredi 23 février 2024 de 11h à 12h30 (GMT+1)
Moscou / Dubaï : Vendredi 23 février 2024 de 13h à 14h30 (GMT+1)
Bangkok : Vendredi 23 février 2024 de 16h à 17h30 (GMT+1)
Shanghai : Vendredi 23 février 2024 de 17h à 18h30 (GMT+1)
Tokyo : Vendredi 23 février 2024 de 18h à 19h30 (GMT+1)
Sydney : Vendredi 23 février 2024 de 19h à 20h30 (GMT+1)
Wellington : Vendredi 23 février 2024 de 21h à 22h30 (GMT+1)
San Francisco : Vendredi 23 février 2024 de 01h à 02h30 (GMT+1)
New York : Vendredi 23 février 2024 de 04h à 05h30 (GMT+1)
Buenos Aires : Vendredi 23 février 2024 de 06h à 07h30 (GMT+1)
London / Dublin : Vendredi 23 février 2024 de 09h à 10h30 (GMT+1)
Paris / Berlin : Vendredi 23 février 2024 de 10h à 11h30 (GMT+1)
Istanbul : Vendredi 23 février 2024 de 11h à 12h30 (GMT+1)
Moscou / Dubaï : Vendredi 23 février 2024 de 13h à 14h30 (GMT+1)
Bangkok : Vendredi 23 février 2024 de 16h à 17h30 (GMT+1)
Shanghai : Vendredi 23 février 2024 de 17h à 18h30 (GMT+1)
Tokyo : Vendredi 23 février 2024 de 18h à 19h30 (GMT+1)
Sydney : Vendredi 23 février 2024 de 19h à 20h30 (GMT+1)
Wellington : Vendredi 23 février 2024 de 21h à 22h30 (GMT+1)
Realizing the PAD Workflow of the Future
A more efficient and Versatile Approach to Complex Lesions
Partager
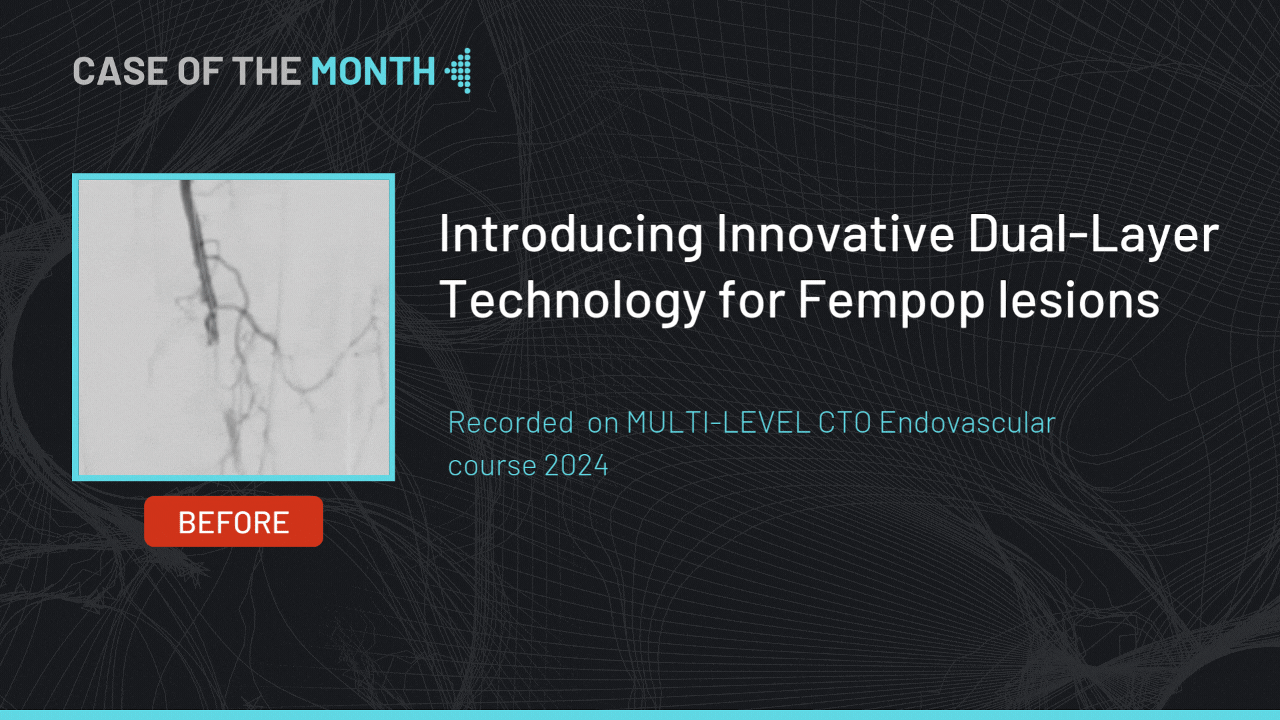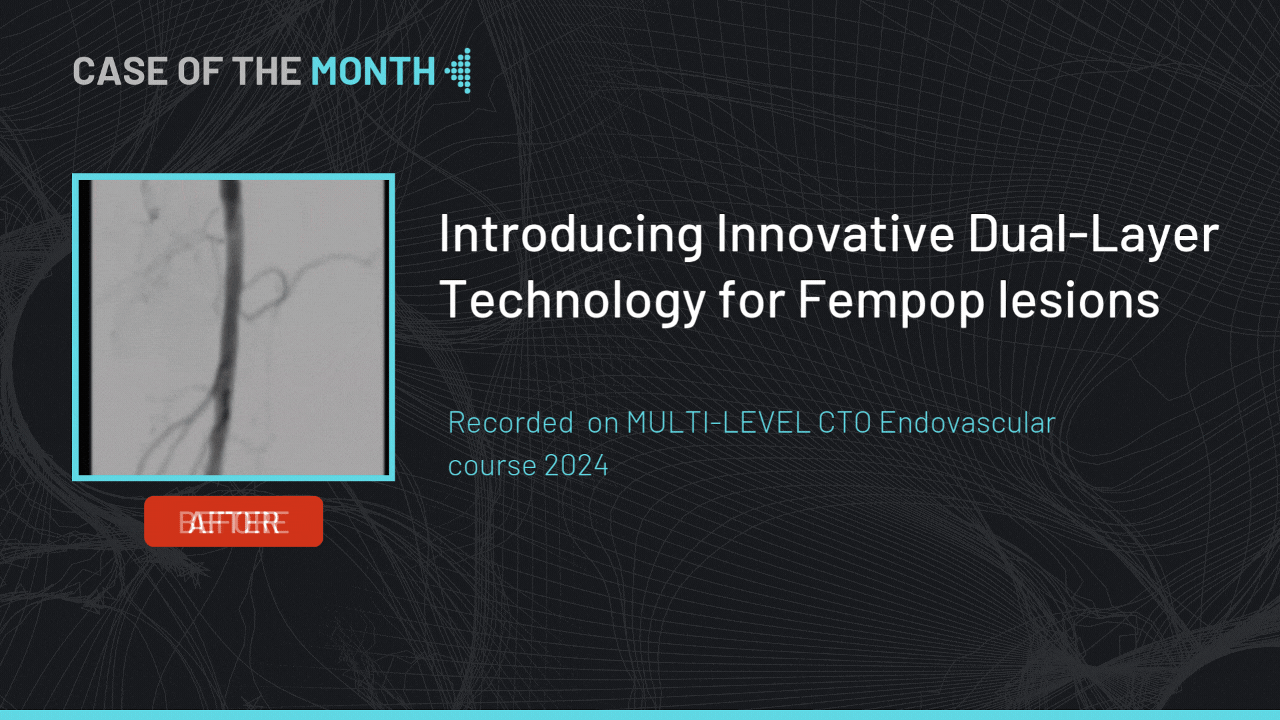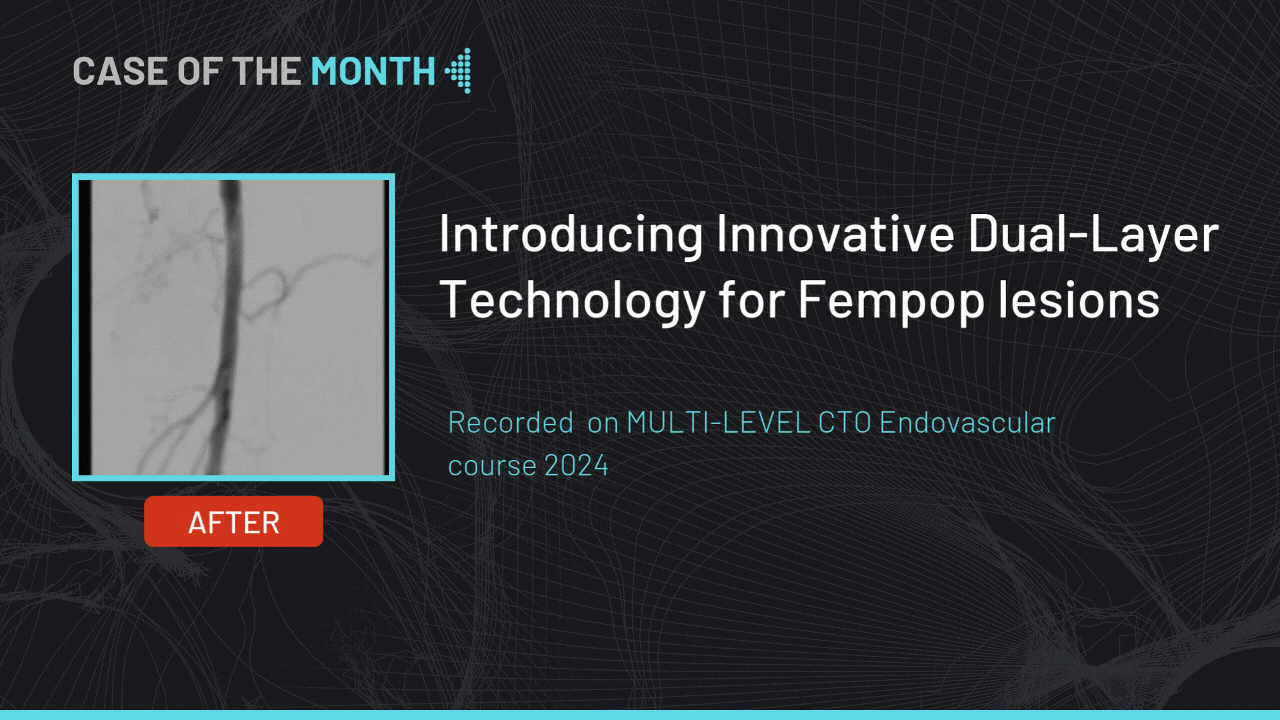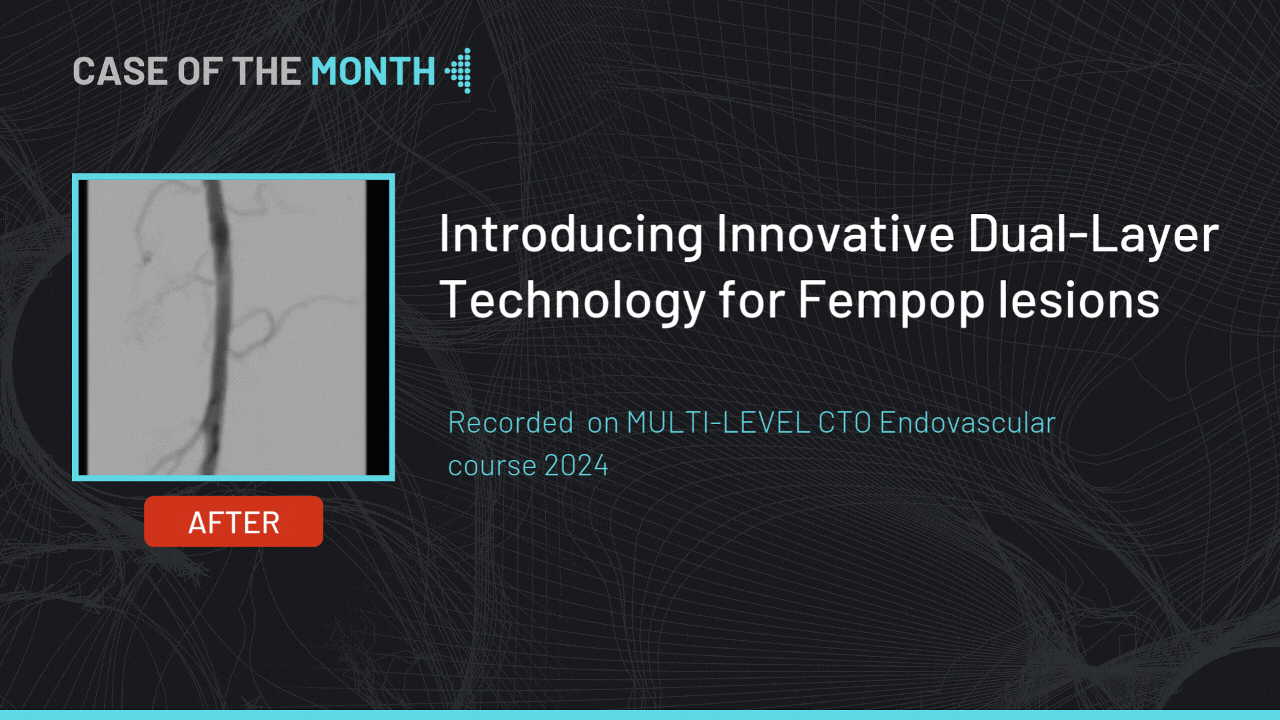
Introducing Innovative Dual-Layer Technology for Fempop lesions
Case of the month : December 2024
Partager
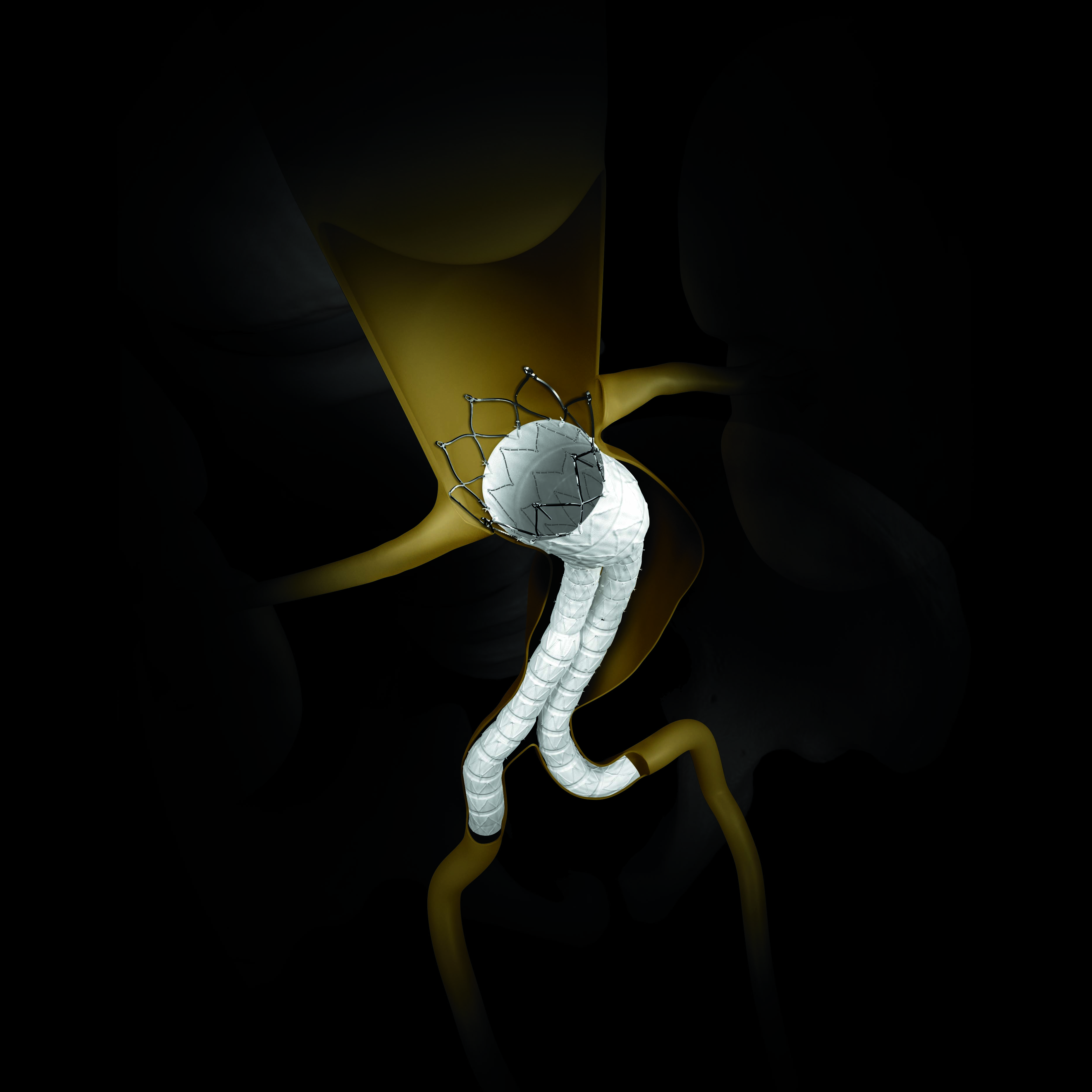
Bifurcated stent graft Endurant II .Tortuous iliac arteries .Angulated neck .Accessory left Renal ar...
Femoral closing - From St. Franziskus Hospital, Munster, Germany
Partager









Martin R. Thanks for your presentation. Do you believe an RCT is needed to demonstrate benefits of PEVAR vs opening the groin?
Enrique P. the overlapping zone...should be the same in both limbs ??
José Antonio D. Sorry for not answering your question in the live session. Overlapping can be adjusted independently in each limb. The ipsilateral limb is approximately 1cm longer than the contralateral limb. From the end of each limb there is a 2 cm minimum overlapping for both, and other 3 cm proximally in the ipsilateral limb (5 cm total) / 2 cm contralateral (4 cm total), with which you can "play" and adjust the length as necessary.
Un abrazo desde Oviedo.
Angelo D. Very good procedure! Yesterday we performed two PEVAR using Incraft. I think it's better same limbs overlapping zone in case of excessive aortic angles at the level of the distal portion of the main body
Alexandre Coutinho . Thank you to share the cases. Best regards