×
It looks like you're using an obsolete version of internet explorer. Internet explorer is no longer supported by Microsoft since the end of 2015. We invite you to use a newer browser such as Firefox, Google Chrome or Microsoft Edge.
My Player placeholder

Become an Incathlab member and receive full access to its content!
You must be an Incathlab member to access videos without any restrictions. Register for free in one minute and access all services provided by Incathlab.You will also be able to log into Incathlab from your Facebook or twitter account by clicking on login on the top-right corner of Incathlab website.
Registration Login
Registration Login
35880 views
The benefits of lower-profile devices are obvious. Not only can more patients with smaller access vessels be treated without the need for conduit access or bypass graft surgery, but access site complications are lower the smaller the device, and more can be done percutaneously without the need for surgical arteriotomy for access.
Discover in this webinar the ultra-low profile abdominal aortic aneurysm stent graft system, which demonstrated to be easy to use, accurate in placement and effective in navigational challenges.
Program
| 12:30 | Product Review & 5 years Innovation study results - Dr Llaneza |
| 12:45 |
1st Case "Narrow access case" - Panel Discussion Dr Llaneza, Dr del Castro and Dr Alonso |
| 13:15 |
Incraft® Concept: an advantage ONLY for complex access? - Dr del Castro Preserve Hypogastric - IN Situ Sizing Short Necks - Deployment accuracy |
| 13:25 |
2nd Case"Hypogastric preservation case" - Panel Discussion Dr Llaneza, Dr del Castro and Dr Alonso |
| 13:55 | Closing & Remarks |
Educational objectives
- Discuss current use of ultra-low profile Incraft® AAA stent graft systems
- Learn from expert discussion of the patients’ outcomes with low profile Incraft® AAA stent graft system treatment
- Demonstrate advanced techniques in the treatment of aortic disease
- Demonstrate new tools available
- Provide platform for discussion and mutual learning
Bibliography
- INNOVATION: Four-year Safety and Effectiveness of the INCRAFT AAA Stent Graft for Endovascular Repair of Abdominal Aortic Aneurysms. Pratesi G., Pratesi C., Chiesa R., Coppi G., Scheinert D., Brunkwall JS., Van der Meulen S., Torsello G.
- Fast-track endovascular aneurysm repair: rationale and design of the multicenter Least Invasive Fast-Track EVAR (LIFE) registry. Krajcer Z., Ramaiah V., Huetter M.
- The Preclose Technique in Percutaneous Endovascular Aortic Repair: A Systematic Literature Review and Meta-Analysis. Jaffan AA., Prince EA., Hampson CO., Murphy TP.
- A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). Nelson PR., Kracjer Z., Kansal N., Rao V., Bianchi C., Hashemi H., Jones P., Bacharach JM.
Audience
- Endovascular specialists (Vascular Surgeons, Interventional Radiologists, Interventional Angiologists and Interventional Cardiologists)
-
Referring physicians of patients with vascular disease
IMPORTANT NOTICE
This webinar is an educational event supported by Cordis Corporation, and is intended for Healthcare Professionals in Europe Middle East and Africa.
The use of the INCRAFT® AAA Stent-Graft System requires that physicians be specially trained in endovascular abdominal aortic aneurysm repair techniques, including experience with high resolution fluoroscopy and radiation safety. Cordis Corporation will provide training specific to the INCRAFT® AAA Stent-Graft System.
The INCRAFT® System is currently approved for investigational device use only in the U.S. and Japan and is being studied in a global pivotal clinical study in the U.S. and Japan called the INSPIRATION Trial, which completed enrollment in 2013.
While every effort is made to see that no inaccurate or misleading data, opinions, or statements appear in this webinar, Cordis Corporation wish to make it clear that material contained in the webinar represents independent evaluations and opinions of the authors and contributors. As a consequence, Cordis Corporation accepts no responsibility for the consequences of any such inaccurate or misleading data or statements. Neither do they endorse the content or the use of any drug or medical device in a way that lies outside its current licensed application in any territory. This presentation may include the demonstration of the use of medical devices; it is not intended to be used as a training guide. The steps demonstrated may not be the complete steps of the procedure. Individual preference and experience, as well as patient needs, may dictate variation in procedure steps. Before using any medical device, including those demonstrated or referenced in this webinar, review all relevant package inserts, with particular attention to the indications, contraindications, warnings and precautions, and steps for use of the device.
All content in these presentations is owned by their authors and is protected by worldwide copyright laws. No modification or further reproduction of the content is permitted.
Dr Manuel Alonso, Dr Llaneza and Dr Del Castro are compensated by and presenting on behalf of Cordis.
For Healthcare Professionals Only.
©2017 Cardinal Health.
Cordis, Cordis LOGO and INCRAFT are trademarks or registered trademarks of Cardinal Health.
EU2321 05/17
Shooting date : 2017-05-24
Last update : 2017-08-29
Last update : 2017-08-29
Join the Discussion
Suggestions
Wednesday, October 23rd 2024 from 12pm to 12:30pm (GMT+2)
Honolulu : Wednesday, October 23rd 2024 from 12am to 12:30am (GMT+2)
San Francisco : Wednesday, October 23rd 2024 from 03am to 03:30am (GMT+2)
New York : Wednesday, October 23rd 2024 from 06am to 06:30am (GMT+2)
Buenos Aires : Wednesday, October 23rd 2024 from 07am to 07:30am (GMT+2)
Reykjavik : Wednesday, October 23rd 2024 from 10am to 10:30am (GMT+2)
London / Dublin : Wednesday, October 23rd 2024 from 11am to 11:30am (GMT+2)
Paris / Berlin : Wednesday, October 23rd 2024 from 12pm to 12:30pm (GMT+2)
Istanbul : Wednesday, October 23rd 2024 from 01pm to 01:30pm (GMT+2)
Moscou / Dubaï : Wednesday, October 23rd 2024 from 02pm to 02:30pm (GMT+2)
Bangkok : Wednesday, October 23rd 2024 from 05pm to 05:30pm (GMT+2)
Shanghai : Wednesday, October 23rd 2024 from 06pm to 06:30pm (GMT+2)
Tokyo : Wednesday, October 23rd 2024 from 07pm to 07:30pm (GMT+2)
Sydney : Wednesday, October 23rd 2024 from 09pm to 09:30pm (GMT+2)
Wellington : Wednesday, October 23rd 2024 from 11pm to 11:30pm (GMT+2)
San Francisco : Wednesday, October 23rd 2024 from 03am to 03:30am (GMT+2)
New York : Wednesday, October 23rd 2024 from 06am to 06:30am (GMT+2)
Buenos Aires : Wednesday, October 23rd 2024 from 07am to 07:30am (GMT+2)
Reykjavik : Wednesday, October 23rd 2024 from 10am to 10:30am (GMT+2)
London / Dublin : Wednesday, October 23rd 2024 from 11am to 11:30am (GMT+2)
Paris / Berlin : Wednesday, October 23rd 2024 from 12pm to 12:30pm (GMT+2)
Istanbul : Wednesday, October 23rd 2024 from 01pm to 01:30pm (GMT+2)
Moscou / Dubaï : Wednesday, October 23rd 2024 from 02pm to 02:30pm (GMT+2)
Bangkok : Wednesday, October 23rd 2024 from 05pm to 05:30pm (GMT+2)
Shanghai : Wednesday, October 23rd 2024 from 06pm to 06:30pm (GMT+2)
Tokyo : Wednesday, October 23rd 2024 from 07pm to 07:30pm (GMT+2)
Sydney : Wednesday, October 23rd 2024 from 09pm to 09:30pm (GMT+2)
Wellington : Wednesday, October 23rd 2024 from 11pm to 11:30pm (GMT+2)
The Complex Made Simple: A Novel All-in-One Solution for Infrainguinal CTO
Share
0
Days
0
Hours
0
Minutes
0
Seconds
Friday, April 4th 2025 from 01pm to 03pm (GMT+2)
Honolulu : Friday, April 4th 2025 from 01am to 03am (GMT+2)
San Francisco : Friday, April 4th 2025 from 04am to 06am (GMT+2)
New York : Friday, April 4th 2025 from 07am to 09am (GMT+2)
Buenos Aires : Friday, April 4th 2025 from 08am to 10am (GMT+2)
Reykjavik : Friday, April 4th 2025 from 11am to 01pm (GMT+2)
London / Dublin : Friday, April 4th 2025 from 12pm to 02pm (GMT+2)
Paris / Berlin : Friday, April 4th 2025 from 01pm to 03pm (GMT+2)
Istanbul : Friday, April 4th 2025 from 02pm to 04pm (GMT+2)
Moscou / Dubaï : Friday, April 4th 2025 from 03pm to 05pm (GMT+2)
Bangkok : Friday, April 4th 2025 from 06pm to 08pm (GMT+2)
Shanghai : Friday, April 4th 2025 from 07pm to 09pm (GMT+2)
Tokyo : Friday, April 4th 2025 from 08pm to 10pm (GMT+2)
Sydney : Friday, April 4th 2025 from 10pm to 12am (GMT+2)
Wellington : Saturday, April 5th 2025 from 12am to 02am (GMT+2)
San Francisco : Friday, April 4th 2025 from 04am to 06am (GMT+2)
New York : Friday, April 4th 2025 from 07am to 09am (GMT+2)
Buenos Aires : Friday, April 4th 2025 from 08am to 10am (GMT+2)
Reykjavik : Friday, April 4th 2025 from 11am to 01pm (GMT+2)
London / Dublin : Friday, April 4th 2025 from 12pm to 02pm (GMT+2)
Paris / Berlin : Friday, April 4th 2025 from 01pm to 03pm (GMT+2)
Istanbul : Friday, April 4th 2025 from 02pm to 04pm (GMT+2)
Moscou / Dubaï : Friday, April 4th 2025 from 03pm to 05pm (GMT+2)
Bangkok : Friday, April 4th 2025 from 06pm to 08pm (GMT+2)
Shanghai : Friday, April 4th 2025 from 07pm to 09pm (GMT+2)
Tokyo : Friday, April 4th 2025 from 08pm to 10pm (GMT+2)
Sydney : Friday, April 4th 2025 from 10pm to 12am (GMT+2)
Wellington : Saturday, April 5th 2025 from 12am to 02am (GMT+2)
The Drug Eluting Technology Show
Share
Friday, February 23rd 2024 from 11am to 12:30pm (GMT+1)
Honolulu : Thursday, February 22nd 2024 from 11pm to 12:30am (GMT+1)
San Francisco : Friday, February 23rd 2024 from 01am to 02:30am (GMT+1)
New York : Friday, February 23rd 2024 from 04am to 05:30am (GMT+1)
Buenos Aires : Friday, February 23rd 2024 from 06am to 07:30am (GMT+1)
London / Dublin : Friday, February 23rd 2024 from 09am to 10:30am (GMT+1)
Paris / Berlin : Friday, February 23rd 2024 from 10am to 11:30am (GMT+1)
Istanbul : Friday, February 23rd 2024 from 11am to 12:30pm (GMT+1)
Moscou / Dubaï : Friday, February 23rd 2024 from 01pm to 02:30pm (GMT+1)
Bangkok : Friday, February 23rd 2024 from 04pm to 05:30pm (GMT+1)
Shanghai : Friday, February 23rd 2024 from 05pm to 06:30pm (GMT+1)
Tokyo : Friday, February 23rd 2024 from 06pm to 07:30pm (GMT+1)
Sydney : Friday, February 23rd 2024 from 07pm to 08:30pm (GMT+1)
Wellington : Friday, February 23rd 2024 from 09pm to 10:30pm (GMT+1)
San Francisco : Friday, February 23rd 2024 from 01am to 02:30am (GMT+1)
New York : Friday, February 23rd 2024 from 04am to 05:30am (GMT+1)
Buenos Aires : Friday, February 23rd 2024 from 06am to 07:30am (GMT+1)
London / Dublin : Friday, February 23rd 2024 from 09am to 10:30am (GMT+1)
Paris / Berlin : Friday, February 23rd 2024 from 10am to 11:30am (GMT+1)
Istanbul : Friday, February 23rd 2024 from 11am to 12:30pm (GMT+1)
Moscou / Dubaï : Friday, February 23rd 2024 from 01pm to 02:30pm (GMT+1)
Bangkok : Friday, February 23rd 2024 from 04pm to 05:30pm (GMT+1)
Shanghai : Friday, February 23rd 2024 from 05pm to 06:30pm (GMT+1)
Tokyo : Friday, February 23rd 2024 from 06pm to 07:30pm (GMT+1)
Sydney : Friday, February 23rd 2024 from 07pm to 08:30pm (GMT+1)
Wellington : Friday, February 23rd 2024 from 09pm to 10:30pm (GMT+1)
Realizing the PAD Workflow of the Future
A more efficient and Versatile Approach to Complex Lesions
Share
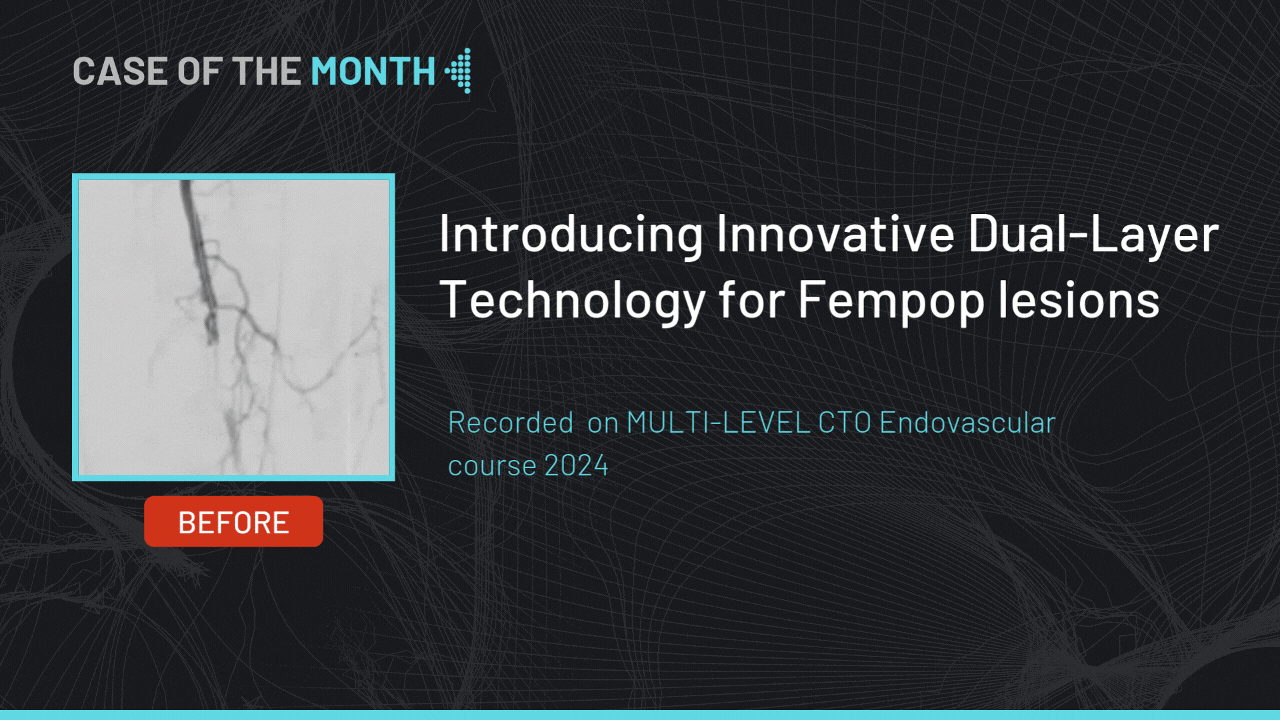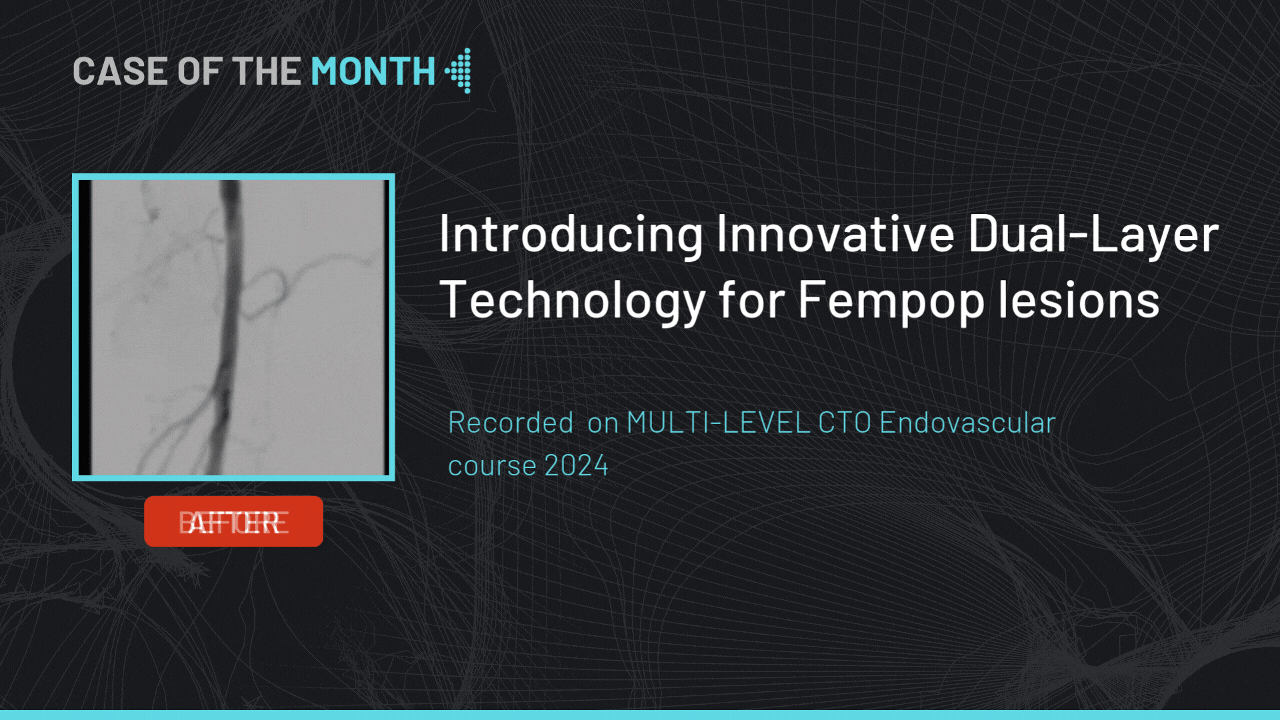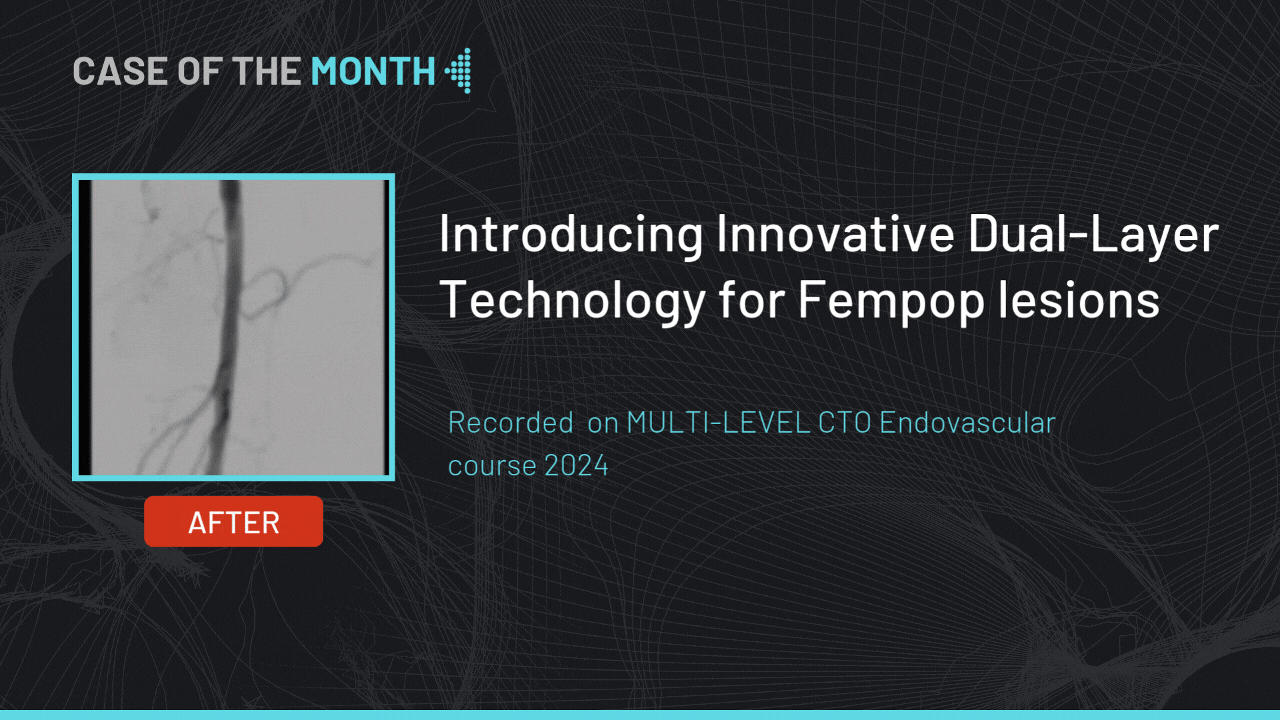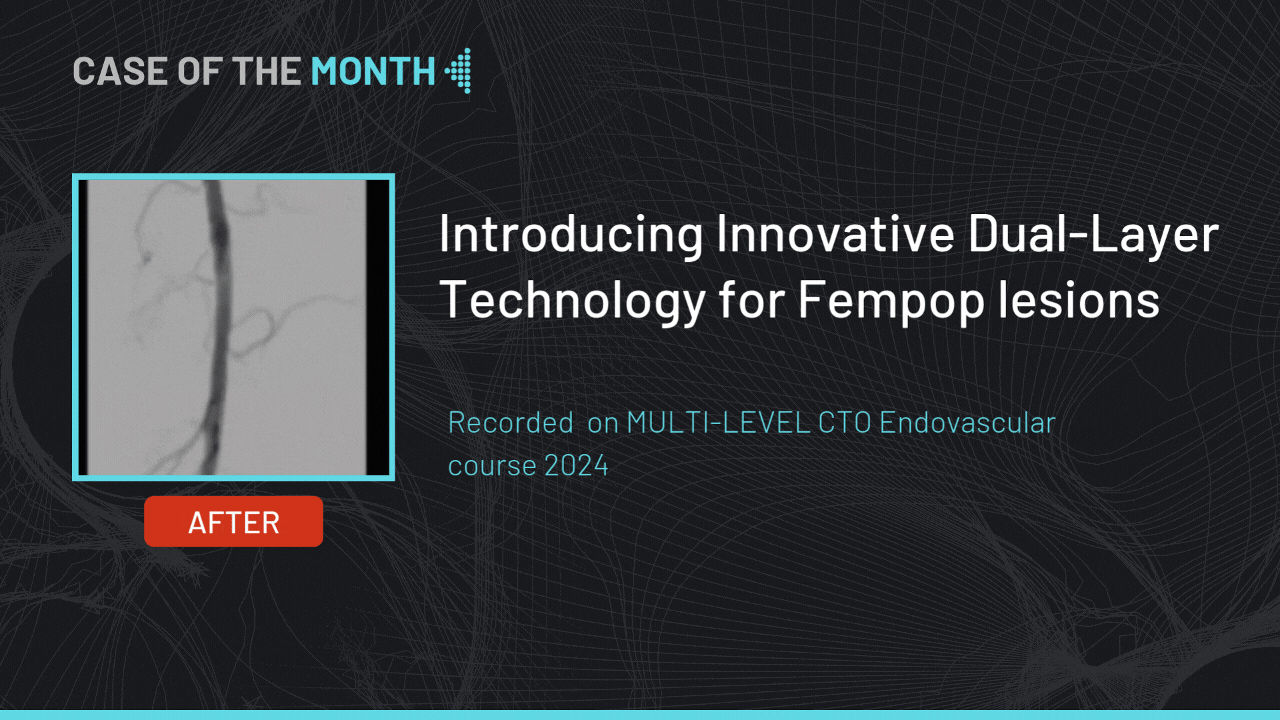
Introducing Innovative Dual-Layer Technology for Fempop lesions
Case of the month : December 2024
Share
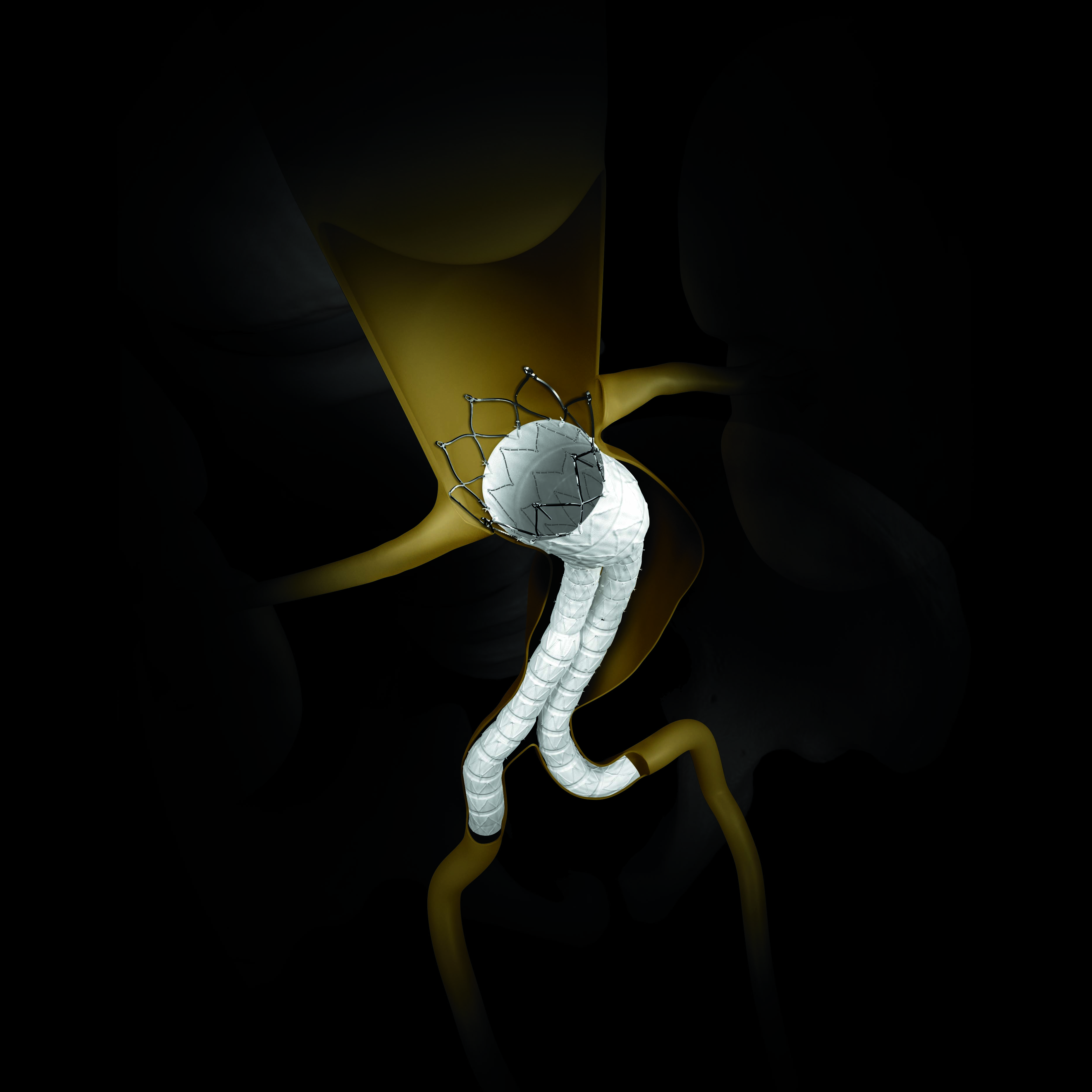
Bifurcated stent graft Endurant II .Tortuous iliac arteries .Angulated neck .Accessory left Renal ar...
Femoral closing - From St. Franziskus Hospital, Munster, Germany
Share










Martin R. Thanks for your presentation. Do you believe an RCT is needed to demonstrate benefits of PEVAR vs opening the groin?
Enrique P. the overlapping zone...should be the same in both limbs ??
José Antonio D. Sorry for not answering your question in the live session. Overlapping can be adjusted independently in each limb. The ipsilateral limb is approximately 1cm longer than the contralateral limb. From the end of each limb there is a 2 cm minimum overlapping for both, and other 3 cm proximally in the ipsilateral limb (5 cm total) / 2 cm contralateral (4 cm total), with which you can "play" and adjust the length as necessary.
Un abrazo desde Oviedo.
Angelo D. Very good procedure! Yesterday we performed two PEVAR using Incraft. I think it's better same limbs overlapping zone in case of excessive aortic angles at the level of the distal portion of the main body
Alexandre Coutinho . Thank you to share the cases. Best regards